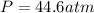Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
A 1.93-mol sample of xenon gas is maintained in a 0.805-L container at 306 K. Calculate the pressure...
Questions

Mathematics, 24.02.2021 19:20

Mathematics, 24.02.2021 19:20


Mathematics, 24.02.2021 19:20




Mathematics, 24.02.2021 19:20


Mathematics, 24.02.2021 19:20




Mathematics, 24.02.2021 19:20

Mathematics, 24.02.2021 19:20

English, 24.02.2021 19:20

Mathematics, 24.02.2021 19:20



Mathematics, 24.02.2021 19:20

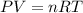
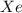 gas = ?
gas = ?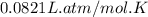
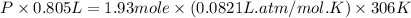
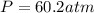
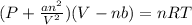
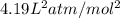
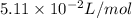
![(P+\frac{(4.19L^2atm/mol^2)\times (1.93mole)^2}{(0.805L)^2})[0.805L-(1.93mole)\times (5.11\times 10^{-2}L/mol)]=1.93mole\times (0.0821L.atm/mol.K)\times 306K](/tpl/images/0566/1070/6fd78.png)