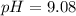Chemistry, 26.03.2020 23:02 chambless1828
A chemist titrates 220.0 mL of a 0.1917M propionic acid (HC2H5CO2) solution with 0.1787 M KOH solution at 25°C. Calculate the pH at equivalence. The pKa of propionic acid is 4.89.
Round your answer to 2 decimal places.

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
A chemist titrates 220.0 mL of a 0.1917M propionic acid (HC2H5CO2) solution with 0.1787 M KOH soluti...
Questions

English, 27.09.2019 00:10

Social Studies, 27.09.2019 00:10

Mathematics, 27.09.2019 00:10

















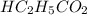 = 0.1917 M
= 0.1917 M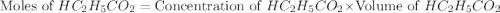
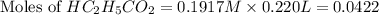
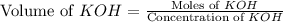
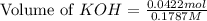
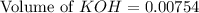
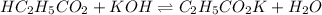
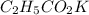 = 0.0422 mol
= 0.0422 mol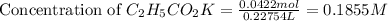
![pH=\frac{1}{2}[pK_w+pK_a+\log C]](/tpl/images/0566/1729/b44e5.png)
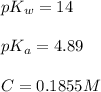
![pH=\frac{1}{2}[14+4.89+\log (0.1855)]](/tpl/images/0566/1729/89494.png)