
Chemistry, 26.03.2020 23:53 melanyaguirre25
What mass of hydrogen peroxide must be used to produce 1.00 L of oxygen at 25.0°C and 1.00 ATM?
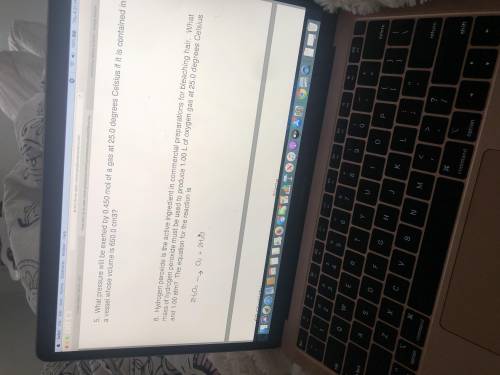

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
What mass of hydrogen peroxide must be used to produce 1.00 L of oxygen at 25.0°C and 1.00 ATM?
Questions


Mathematics, 21.09.2020 14:01

Chemistry, 21.09.2020 14:01

English, 21.09.2020 14:01

English, 21.09.2020 14:01

Computers and Technology, 21.09.2020 14:01

Geography, 21.09.2020 14:01

Mathematics, 21.09.2020 14:01


Spanish, 21.09.2020 14:01



Computers and Technology, 21.09.2020 14:01

Mathematics, 21.09.2020 14:01


World Languages, 21.09.2020 14:01







