
Chemistry, 27.03.2020 01:29 mcckenziee
When the oxide of generic metal M is heated at 25.0 ∘ C , a negligible amount of M is produced. MO 2 ( s ) − ⇀ ↽ − M ( s ) + O 2 ( g ) Δ G ∘ = 291.0 kJ mol When this reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. What is the chemical equation of this coupled process? Show that the reaction is in equilibrium. Include physical states and represent graphite as C ( s ) .

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which statement is a reason to support population regulation? a) it is unethical for us to control birth control rates b) humans have the freedom to produce as many children as desired c) the gap between the rich and poor has been narrowing since 1960 d) billions more people on the earth will intensify many environmental and social problems
Answers: 1


Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

You know the right answer?
When the oxide of generic metal M is heated at 25.0 ∘ C , a negligible amount of M is produced. MO 2...
Questions

Spanish, 02.07.2019 03:30





Arts, 02.07.2019 03:30

Mathematics, 02.07.2019 03:30

Biology, 02.07.2019 03:30

Mathematics, 02.07.2019 03:30


Chemistry, 02.07.2019 03:30


Mathematics, 02.07.2019 03:30

Mathematics, 02.07.2019 03:30






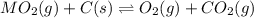
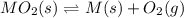 .....[1]
.....[1]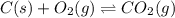 ..[2]
..[2]

