
Chemistry, 27.03.2020 01:50 marsewilliams
Suppose the reaction system below has already reached equilibrium. Predict the effect that each of the following changes will have on the equilibrium position. Tell whether the equilibrium will shift to the right, will shift to the left, or will not be affected.
UO2(s) + 4 HF(g) ↔ UF4(g) + 2 H2O(g)
1. Water vapor is removed
2. UF4(g) is added
3. The reaction is done in a glass reaction vessel. HF(g) attacks and reacts with the glass
4. If the reaction is endothermic, and you want to make the equilibrium shift to the right to maximize the products, would you heat or cool the reaction vessel?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
Suppose the reaction system below has already reached equilibrium. Predict the effect that each of t...
Questions






Mathematics, 29.06.2019 10:30



Mathematics, 29.06.2019 10:30




Biology, 29.06.2019 10:30




Social Studies, 29.06.2019 10:30

Social Studies, 29.06.2019 10:30


Computers and Technology, 29.06.2019 10:30

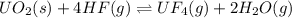
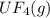 is added
is added

