
Chemistry, 27.03.2020 03:10 drivinghydra
A 0.0900 g sample of household bleach is completely reacted with KI(s). The resulting solution is then titrated with 0.150 M NaS2O3 solution. 0.750 mL of the solution is required to reach the colorless endpoint. What is the mass percent of NaOCl (MM = 74.44 g/mole) in the bleach?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

You know the right answer?
A 0.0900 g sample of household bleach is completely reacted with KI(s). The resulting solution is th...
Questions

Mathematics, 14.05.2021 14:00


History, 14.05.2021 14:00

Mathematics, 14.05.2021 14:00

Mathematics, 14.05.2021 14:00


Mathematics, 14.05.2021 14:00





Mathematics, 14.05.2021 14:00


Biology, 14.05.2021 14:00



Mathematics, 14.05.2021 14:00

Computers and Technology, 14.05.2021 14:00



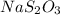 is required for complete neutralization.
is required for complete neutralization.

