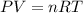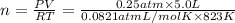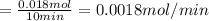Calcium oxide (CaO), an important ingredient in cement, is produced by decomposing calcium carbonate (CaCO3) at high temperature: CaCO3(s) → CaO(s) + CO2(g) In one particular reaction, 25 g of CaCO3 is heated at 550°C in a 5.0 L vessel. The pressure of CO2 is 0.25 atm after 10.0 minutes. What is the average rate of CO2 production in moles per minute during the 10 minutes? (Enter in mol/min.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2
You know the right answer?
Calcium oxide (CaO), an important ingredient in cement, is produced by decomposing calcium carbonate...
Questions

History, 05.09.2020 23:01








Mathematics, 05.09.2020 23:01


Computers and Technology, 05.09.2020 23:01


English, 05.09.2020 23:01

Mathematics, 05.09.2020 23:01


Mathematics, 05.09.2020 23:01


Biology, 05.09.2020 23:01