
Chemistry, 27.03.2020 16:45 janahiac09
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?
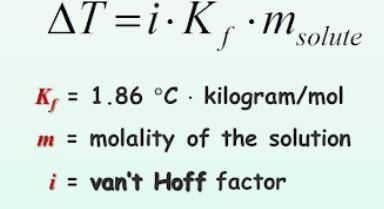

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

You know the right answer?
What is the change in freezing point (delta T) of an aqueous solution that is 0.082 molality AlCl3?<...
Questions

Social Studies, 03.05.2021 20:50



Mathematics, 03.05.2021 20:50

History, 03.05.2021 20:50

Biology, 03.05.2021 20:50

English, 03.05.2021 20:50


Mathematics, 03.05.2021 20:50

Social Studies, 03.05.2021 20:50





Chemistry, 03.05.2021 20:50

Mathematics, 03.05.2021 20:50


Biology, 03.05.2021 20:50


Health, 03.05.2021 20:50



