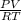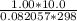Chemistry, 27.03.2020 18:26 ebookhardt917
A 4.00g sample of helium has a volume of 24.4L at a temperature of 25.0oC and a pressure of 1.00 atm. The volume of the helium is reduced to 10.4L, but the temperature and pressure of the gas are kept con- stant. What is the new quantity of the gas, in moles? Show all work using units and sig figs.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
A 4.00g sample of helium has a volume of 24.4L at a temperature of 25.0oC and a pressure of 1.00 atm...
Questions

Arts, 23.09.2020 01:01


Health, 23.09.2020 01:01


Social Studies, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01

Mathematics, 23.09.2020 01:01




Mathematics, 23.09.2020 01:01


Mathematics, 23.09.2020 01:01

Geography, 23.09.2020 01:01


English, 23.09.2020 01:01

Chemistry, 23.09.2020 01:01

Social Studies, 23.09.2020 01:01

Biology, 23.09.2020 01:01