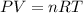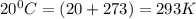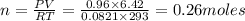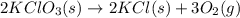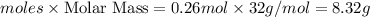Oxygen gas can be prepared by heating potassium chlorate according to the following equation:
<...

Oxygen gas can be prepared by heating potassium chlorate according to the following equation:
2KClO3(s) → 2KCl(s) + 3O2(g)
The product gas, O2, is collected over water at a temperature of 20 °C and a pressure of 747 mm Hg. If the wet O2 gas formed occupies a volume of 6.42 L, the number of grams of O2 formed is g. The vapor pressure of water is 17.5 mm Hg at 20 °C.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
Questions



Mathematics, 02.08.2019 02:30





History, 02.08.2019 02:30

Mathematics, 02.08.2019 02:30


Mathematics, 02.08.2019 02:30


Social Studies, 02.08.2019 02:30


History, 02.08.2019 02:30




Mathematics, 02.08.2019 02:30

World Languages, 02.08.2019 02:30

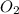 formed is 8.32 g
formed is 8.32 g