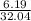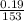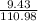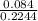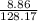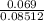Chemistry, 27.03.2020 19:31 carlosleblanc26
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methanol (CH3OH) in 1.50 × 102 mL of solution:
mol/L
(b) 9.43 g of calcium chloride (CaCl2) in 2.20 × 102 mL of solution:
mol/L
(c) 8.86 g of naphthalene (C10H8) in 85.2 mL of benzene solution:
mol/L

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Calculate the molarity of each of the following solutions. (mol/L)
(a) 6.19 g of methano...
(a) 6.19 g of methano...
Questions



English, 03.12.2020 22:00

Mathematics, 03.12.2020 22:00






Mathematics, 03.12.2020 22:00






Mathematics, 03.12.2020 22:00



Mathematics, 03.12.2020 22:00

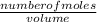 equation 1
equation 1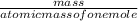 equation 2
equation 2