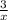Chemistry, 27.03.2020 20:32 kimbyrleeclark
2 points
SC Johnson uses ammonia in Windex. In 1 bottle of Windex, there is 51.0
grams of ammonia (NH3). How many grams of hydrogen gas (H2) will SC
Johnson need to react in order to make 1 bottle of Windex?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
2 points
SC Johnson uses ammonia in Windex. In 1 bottle of Windex, there is 51.0
grams o...
SC Johnson uses ammonia in Windex. In 1 bottle of Windex, there is 51.0
grams o...
Questions



English, 08.04.2021 17:10

Mathematics, 08.04.2021 17:10


Physics, 08.04.2021 17:10


English, 08.04.2021 17:10




Social Studies, 08.04.2021 17:10


Mathematics, 08.04.2021 17:10




Spanish, 08.04.2021 17:10


Arts, 08.04.2021 17:10

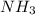
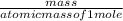
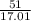
 =
=