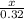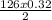Chemistry, 27.03.2020 20:59 kyrabrown33
How many kJ of heat are released by the reaction of 25.0 g of Na2O2(s) in the following reaction? (M = 78.0 g/mol for Na2O2)
2 Na2O2(s) + 2 H2O(l) → 4 NaOH(aq) + O2(g) ∆Hο = −126 kJ

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2



Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
How many kJ of heat are released by the reaction of 25.0 g of Na2O2(s) in the following reaction? (M...
Questions


English, 04.05.2021 18:00




Chemistry, 04.05.2021 18:00

History, 04.05.2021 18:00




Mathematics, 04.05.2021 18:00




Biology, 04.05.2021 18:00

Mathematics, 04.05.2021 18:00


Biology, 04.05.2021 18:00


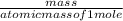

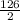 =
=