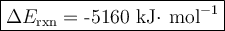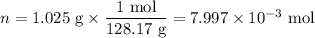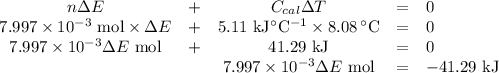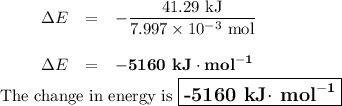Chemistry, 27.03.2020 21:01 usagimiller
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8). When 1.025 gof naphthalene is burned in a bomb calorimeter, the temperature rises from 24.25 ∘C to 32.33 ∘C. Find ΔErxn for the combustion of naphthalene. The heat capacity of the calorimeter, determined in a separate experiment, is 5.11kJ/∘C. Express the change in energy in kilojoules per mole to three significant figures.ΔErxn = kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

You know the right answer?
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8). When 1.025 gof naphthalene...
Questions

Mathematics, 29.03.2020 21:29


Geography, 29.03.2020 21:29

Mathematics, 29.03.2020 21:29


Mathematics, 29.03.2020 21:29

Computers and Technology, 29.03.2020 21:29

Chemistry, 29.03.2020 21:29

Chemistry, 29.03.2020 21:29

Mathematics, 29.03.2020 21:29



Geography, 29.03.2020 21:29



Chemistry, 29.03.2020 21:30


Mathematics, 29.03.2020 21:30


Mathematics, 29.03.2020 21:30