Chemistry, 27.03.2020 22:31 JuniperGalaxy
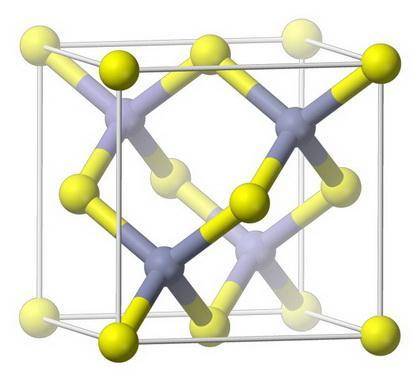
Zinc blende, or sphalerite, can be described as having a face-centered cubic unit cell with sulfur atoms at each lattice point and four zinc atoms within the unit cell. What is the empirical formula for this material? Follow standard convention for writing an empirical formula (ex. for AlCl3 enter AlCl3 not Cl3Al).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Zinc blende, or sphalerite, can be described as having a face-centered cubic unit cell with sulfur a...
Questions



Biology, 23.08.2019 02:30

Mathematics, 23.08.2019 02:30


English, 23.08.2019 02:30

Mathematics, 23.08.2019 02:30

Mathematics, 23.08.2019 02:30


Physics, 23.08.2019 02:30

Health, 23.08.2019 02:30



History, 23.08.2019 02:30

History, 23.08.2019 02:30