Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
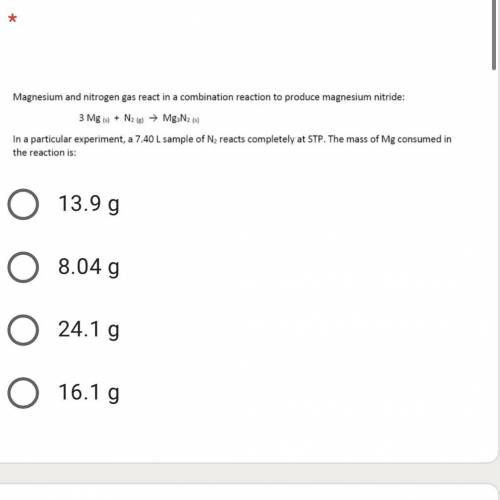
A 7.40 L sample of N2 reacts completely at STP. the mass of the Mg consumed in the reaction is:
Questions

History, 16.07.2019 21:10

SAT, 16.07.2019 21:10

English, 16.07.2019 21:10





Biology, 16.07.2019 21:10



Mathematics, 16.07.2019 21:10



Social Studies, 16.07.2019 21:10

History, 16.07.2019 21:10




History, 16.07.2019 21:10

Chemistry, 16.07.2019 21:20