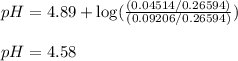Chemistry, 28.03.2020 04:02 cupcake20019peehui
An analytical chemist is titrating of a solution of propionic acid with a solution of 224.9 ml of a 0.6100M solution of propionic acid (HC2H5CO2) with a 1.1000M solution of KOH. The pKa of proionic acid 4.89.
Calculate the pH of the acid solution after the chemist has added 41.04mL of the KOH solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1
You know the right answer?
An analytical chemist is titrating of a solution of propionic acid with a solution of 224.9 ml of a...
Questions







Geography, 06.09.2021 04:40

Social Studies, 06.09.2021 04:40





English, 06.09.2021 04:50

Computers and Technology, 06.09.2021 04:50


English, 06.09.2021 04:50


Chemistry, 06.09.2021 04:50


Chemistry, 06.09.2021 04:50

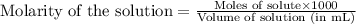 .....(1)
.....(1)
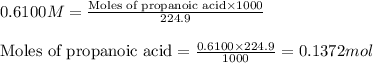
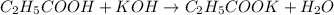
![pH=pK_a+\log(\frac{[\text{salt}]}{[acid]})](/tpl/images/0568/6689/3d096.png)
![pH=pK_a+\log(\frac{[C_2H_5COOK]}{[C_2H_5COOH]})](/tpl/images/0568/6689/3ede9.png)
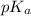 = negative logarithm of acid dissociation constant of propanoic acid = 4.89
= negative logarithm of acid dissociation constant of propanoic acid = 4.89![[C_2H_5COOK]=\frac{0.04514}{0.26594}](/tpl/images/0568/6689/11196.png)
![[C_2H_5COOH]=\frac{0.09206}{0.26594}](/tpl/images/0568/6689/86178.png)