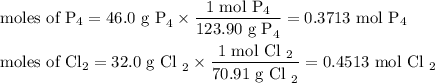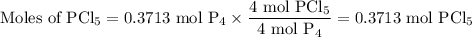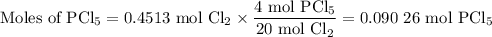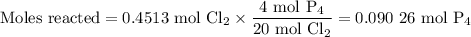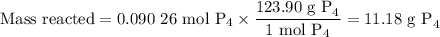Chemistry, 28.03.2020 04:02 shinyelish6
04.05 mol
The reaction of chlorine gas with solid phosphorus (P4) produces solid
phosphorus pentachloride. When 32.0 g of chlorine reacts with 46.0 g of P4.
What is the mass in excess of the excess reactantt?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
04.05 mol
The reaction of chlorine gas with solid phosphorus (P4) produces solid
phospho...
The reaction of chlorine gas with solid phosphorus (P4) produces solid
phospho...
Questions

Mathematics, 12.03.2022 05:20




Biology, 12.03.2022 05:30

Computers and Technology, 12.03.2022 05:30

Mathematics, 12.03.2022 05:30


Social Studies, 12.03.2022 05:30


SAT, 12.03.2022 05:30



Mathematics, 12.03.2022 05:30






Mathematics, 12.03.2022 05:40