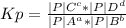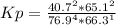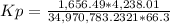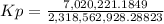Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2(g) → 2 Cl2(g) + 2 H2O(g)
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen chloride, oxygen, chlorine, and water has the following composition:
COMPOUND Pressure at equilibrium
HCl 76.9 atm
O2 66.3 atm
Cl2 40.7 atm
H2O 65.1 atm
Calculate the value of the equilibrium constant Kp for this reaction. Round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3
You know the right answer?
Hydrogen chloride and oxygen react to form chlorine and water, like this:
4 HCl(g) + O2...
4 HCl(g) + O2...
Questions

Biology, 13.10.2020 15:01



Mathematics, 13.10.2020 15:01


Mathematics, 13.10.2020 15:01







Mathematics, 13.10.2020 15:01



Social Studies, 13.10.2020 15:01

English, 13.10.2020 15:01

French, 13.10.2020 15:01