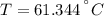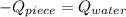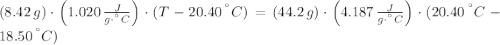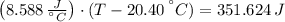Chemistry, 28.03.2020 18:38 veroushkarose7326
An 8.42 g piece of metal with a specific heat of 1.020 J g-1 k-1 wasn't heated to an unknown temperature. The metal was then placed in 44.2 g of water with an initial temperature of 18.50 C. If the final temperature of the water was 20.40 C, what temperature was the metal initially heated to (in C).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
An 8.42 g piece of metal with a specific heat of 1.020 J g-1 k-1 wasn't heated to an unknown tempera...
Questions


Mathematics, 16.10.2021 19:10


SAT, 16.10.2021 19:10

SAT, 16.10.2021 19:10



SAT, 16.10.2021 19:10


Geography, 16.10.2021 19:10

Mathematics, 16.10.2021 19:10

Mathematics, 16.10.2021 19:10

English, 16.10.2021 19:10

Social Studies, 16.10.2021 19:10

Social Studies, 16.10.2021 19:10

Mathematics, 16.10.2021 19:10


Geography, 16.10.2021 19:10

Chemistry, 16.10.2021 19:10

Mathematics, 16.10.2021 19:10