
Chemistry, 28.03.2020 22:53 ExperimentsDIYS
Initially the concentration of H2 is 2.0M , I2 is 2.0M and HI is 8.0M. The vessel is heated up to 675 degrees at equilibrium the concentration of HI changes to become 6.0M. Calculate the value of k at this temperature.

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3
You know the right answer?
Initially the concentration of H2 is 2.0M , I2 is 2.0M and HI is 8.0M. The vessel is heated up to 67...
Questions

Computers and Technology, 14.07.2019 04:22





Computers and Technology, 14.07.2019 04:22

Computers and Technology, 14.07.2019 04:22

Computers and Technology, 14.07.2019 04:22

Computers and Technology, 14.07.2019 04:22

Computers and Technology, 14.07.2019 04:22

Computers and Technology, 14.07.2019 04:22

Computers and Technology, 14.07.2019 04:22

Mathematics, 14.07.2019 04:22





Computers and Technology, 14.07.2019 04:22

Physics, 14.07.2019 04:22

![\frac{[HI]^{2} }{[H]_{2} I_{2} }](/tpl/images/0569/1352/e5ad7.png)
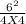
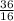 = 2.25
= 2.25

