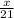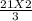Chemistry, 28.03.2020 22:17 NetherisIsTheQueen
Consider the following balanced reaction between hydrogen and nitrogen to form ammonia:
3H2(g)+N2(g)→2NH3(g)
How many moles of NH3 can be produced from 21.0 mol of H2 and excess N2?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1


Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Consider the following balanced reaction between hydrogen and nitrogen to form ammonia:
...
...
Questions

Mathematics, 06.12.2020 22:00

Advanced Placement (AP), 06.12.2020 22:00



Mathematics, 06.12.2020 22:00





English, 06.12.2020 22:00

English, 06.12.2020 22:00

Health, 06.12.2020 22:00

History, 06.12.2020 22:00

Mathematics, 06.12.2020 22:00




Geography, 06.12.2020 22:00

Mathematics, 06.12.2020 22:00

English, 06.12.2020 22:00

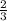 =
=