If a 210.5g sample of phenol (C6H5OH) is isolated in a laboratory how many
moles of hydrogen a...

Chemistry, 29.03.2020 01:30 madiiiiiii69
If a 210.5g sample of phenol (C6H5OH) is isolated in a laboratory how many
moles of hydrogen are within phenol. (C6H5OH=94.11g/mol]
0.372 mol H
13.4 mol H.
11.2 mol H
2.23 mol H

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
Questions


History, 15.10.2019 19:30


Mathematics, 15.10.2019 19:30



Mathematics, 15.10.2019 19:30


Social Studies, 15.10.2019 19:30


Physics, 15.10.2019 19:30

Mathematics, 15.10.2019 19:30

Health, 15.10.2019 19:30


History, 15.10.2019 19:30


History, 15.10.2019 19:30

Mathematics, 15.10.2019 19:30

Business, 15.10.2019 19:30

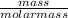
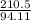 = 2.24mol
= 2.24mol

