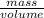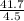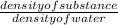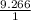Chemistry, 29.03.2020 03:05 alishabhappy1
41.7 g of a substance are added to a graduated flask containing 523 ml of water The
water level rises to the 56.8 ml mark. From this information, calculate the density and
specific gravity

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
41.7 g of a substance are added to a graduated flask containing 523 ml of water The
water leve...
water leve...
Questions



Mathematics, 12.10.2019 12:30

Biology, 12.10.2019 12:30

Biology, 12.10.2019 12:30






Mathematics, 12.10.2019 12:30


Biology, 12.10.2019 12:30

History, 12.10.2019 12:30

Social Studies, 12.10.2019 12:30

English, 12.10.2019 12:30

English, 12.10.2019 12:30

History, 12.10.2019 12:30