
Chemistry, 29.03.2020 04:56 friskowale
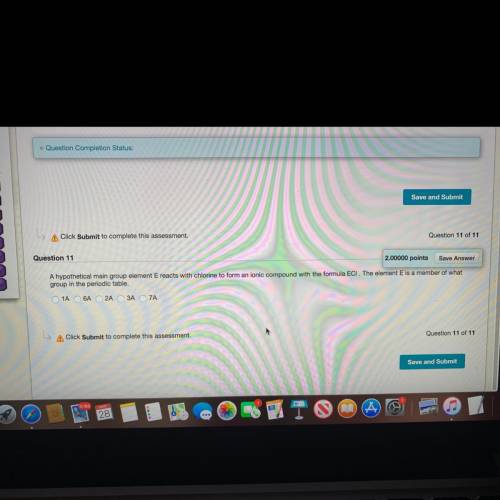
A hypothetical main group element E reacts with chlorine to form an ionic compound with the formula ECl. The element is a member of what group in the periodic table.
1A, 6A, 2A, 3A, or 7A?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1

Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
You know the right answer?
A hypothetical main group element E reacts with chlorine to form an ionic compound with the formula...
Questions

Mathematics, 03.12.2019 16:31


Geography, 03.12.2019 16:31

Geography, 03.12.2019 16:31

Social Studies, 03.12.2019 16:31

Chemistry, 03.12.2019 16:31

Physics, 03.12.2019 16:31


Mathematics, 03.12.2019 16:31


Mathematics, 03.12.2019 16:31


Mathematics, 03.12.2019 16:31



History, 03.12.2019 16:31

History, 03.12.2019 16:31



English, 03.12.2019 16:31



