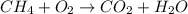Chemistry, 05.01.2020 16:31 murtaghliam1
Given a chemical reaction, methane (ch4) burns in oxygen (o2) to produce carbon dioxide (co2) and water (h2o). which of these represent an unbalanced equation with the correct chemical formulas? . a.. ch4 + o2 co2 + h2o. b. ch4+ co2 o2 + h2o. c. co2+ h2o ch4+ o2. d. ch4 o2 + co2+ h2o

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Given a chemical reaction, methane (ch4) burns in oxygen (o2) to produce carbon dioxide (co2) and wa...
Questions


Mathematics, 04.03.2021 21:50


Mathematics, 04.03.2021 21:50


History, 04.03.2021 21:50

Mathematics, 04.03.2021 21:50


Social Studies, 04.03.2021 21:50

History, 04.03.2021 21:50

Mathematics, 04.03.2021 21:50

English, 04.03.2021 21:50



Spanish, 04.03.2021 21:50

Mathematics, 04.03.2021 21:50




Mathematics, 04.03.2021 21:50