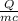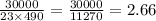30,000 J of heat are added to 23.0 kg of steel to reach a final temperature of 140
°C. What wa...

Chemistry, 29.03.2020 19:47 jennainglish
30,000 J of heat are added to 23.0 kg of steel to reach a final temperature of 140
°C. What was the initial temperature of the steel? (csteel =490J/kg * °C)
1. First find AT using the formula q=mcAT
2. Rearrange to solve for initial temperature using AT=Tfinal -Tinital
I need help quickly

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

You know the right answer?
Questions

History, 28.10.2019 05:31


History, 28.10.2019 05:31



Chemistry, 28.10.2019 05:31

Chemistry, 28.10.2019 05:31


History, 28.10.2019 05:31

Social Studies, 28.10.2019 05:31



Mathematics, 28.10.2019 05:31

History, 28.10.2019 05:31




Mathematics, 28.10.2019 05:31

History, 28.10.2019 05:31

Mathematics, 28.10.2019 05:31