
Chemistry, 29.03.2020 22:17 alligatorsocks
25.0 g of a substance requires 117.4 Joules of energy to raise its temperature from 75.0 to 87.2 oC. What is the specific heat of the substance?
.054 J/g°C
.063 J/g°C
2.60 J/g°C
.385 J/g°C

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1

Chemistry, 23.06.2019 09:30
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2

Chemistry, 23.06.2019 11:00
Asolubility table shows that almost all compounds of group 1 metals are soluble in water. this general rule tells you that mgi2 is soluble rbno3 is soluble cacl2 is soluble co2 is soluble
Answers: 1
You know the right answer?
25.0 g of a substance requires 117.4 Joules of energy to raise its temperature from 75.0 to 87.2 oC....
Questions





Computers and Technology, 17.02.2020 16:58














Computers and Technology, 17.02.2020 17:00

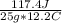 = 0.385 J/g°C
= 0.385 J/g°C

