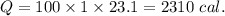Chemistry, 29.03.2020 22:37 Candieboo6939
In lab, a student burns 0.550 grams of Ritz crackers. The burning causes 100.0 grams of water to rise from 24.0°C go 47.1 °C.
Calculate the number of calories absorbed by the WATER as the Ritz crackers burn. (Use the specific heat of water in terms of calories: 1.0 cal/ g °C) ** Remember you are calculating the heat gained by the water, NOT the heat lost by the Ritz cracker - use the correct mass!!
12.7 cal
53.2 cal
2310 cal
9.67 x 105 cal

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
In lab, a student burns 0.550 grams of Ritz crackers. The burning causes 100.0 grams of water to ris...
Questions



Engineering, 04.06.2021 08:00



Mathematics, 04.06.2021 08:00

Mathematics, 04.06.2021 08:10

History, 04.06.2021 08:10



Mathematics, 04.06.2021 08:10


Biology, 04.06.2021 08:10


History, 04.06.2021 08:10

Mathematics, 04.06.2021 08:10


Mathematics, 04.06.2021 08:10

Mathematics, 04.06.2021 08:10

Chemistry, 04.06.2021 08:10