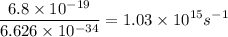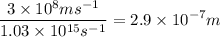Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
An energy of 6.8 x 10^-19 J/atom is required to cause an aluminum atom on a metal surface to lose an...
Questions




Mathematics, 25.02.2020 22:33


Mathematics, 25.02.2020 22:33


Geography, 25.02.2020 22:33

Mathematics, 25.02.2020 22:33


Biology, 25.02.2020 22:33


Mathematics, 25.02.2020 22:33

History, 25.02.2020 22:33



Medicine, 25.02.2020 22:33



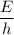 =
=