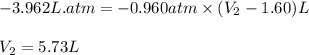Chemistry, 30.03.2020 04:55 ianball025
A sample of flammable liquid is placed into an enclosed cylinder which is then fitted with a movable piston. Initially the cylinder contains a volume of 1.60 L. The sample is ignited producing gas and releasing 401.5 J of energy. To what volume will the cylinder expand to if it must expand against a pressure of 729.8 mmHg. Assume all the energy released is converted to work used to push the piston?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
A sample of flammable liquid is placed into an enclosed cylinder which is then fitted with a movable...
Questions

Mathematics, 14.07.2020 15:01

Mathematics, 14.07.2020 15:01


Mathematics, 14.07.2020 15:01

Chemistry, 14.07.2020 15:01

Mathematics, 14.07.2020 15:01

Mathematics, 14.07.2020 15:01

Mathematics, 14.07.2020 15:01

English, 14.07.2020 15:01


Mathematics, 14.07.2020 15:01


Mathematics, 14.07.2020 15:01

Mathematics, 14.07.2020 15:01

Mathematics, 14.07.2020 15:01

English, 14.07.2020 15:01

Mathematics, 14.07.2020 15:01

Mathematics, 14.07.2020 15:01


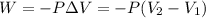
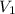 = initial volume = 1.60 L
= initial volume = 1.60 L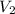 = final volume = ?
= final volume = ?