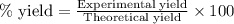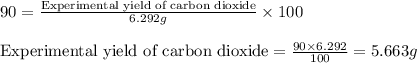Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
Consider the balanced equation for the following reaction:
7O2(g) + 2C2H6(g) → 4CO2(g) + 6H2O(...
7O2(g) + 2C2H6(g) → 4CO2(g) + 6H2O(...
Questions


Mathematics, 26.05.2021 22:00

Mathematics, 26.05.2021 22:00

Mathematics, 26.05.2021 22:00




History, 26.05.2021 22:00


Mathematics, 26.05.2021 22:00





Social Studies, 26.05.2021 22:00

Mathematics, 26.05.2021 22:00

Mathematics, 26.05.2021 22:00

Spanish, 26.05.2021 22:00


Mathematics, 26.05.2021 22:00

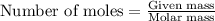 .....(1)
.....(1)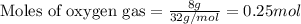
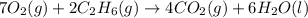
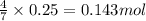 of carbon dioxide
of carbon dioxide