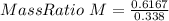Chemistry, 30.03.2020 16:42 ICyberAngel
Chlorine reacts with oxygen to form two compounds. Compound A contains 8.43 g of chlorine for every 13.67 g of oxygen. Compound B contains 5.87 g of chlorine for every 17.33 g of oxygen. What is the mass ratio of chlorine rounded to the nearest whole number

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the reactions in a blast furnace used to reduce iron is shown above. how many grams of fe2o3 are required to produce 15.5 g of fe if the reaction occurs in the presence of excess co? a.11.1 g b.22.1 g c.30.0 g d.44.2 g
Answers: 2

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Chlorine reacts with oxygen to form two compounds. Compound A contains 8.43 g of chlorine for every...
Questions




Biology, 26.12.2019 09:31


Mathematics, 26.12.2019 09:31

History, 26.12.2019 09:31


Mathematics, 26.12.2019 09:31



Social Studies, 26.12.2019 09:31

Mathematics, 26.12.2019 09:31

Mathematics, 26.12.2019 09:31



Mathematics, 26.12.2019 09:31



Social Studies, 26.12.2019 09:31

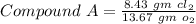
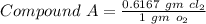 -------- (1)
-------- (1)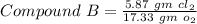 --------- (2)
--------- (2)
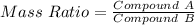 ------ (3)
------ (3)