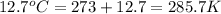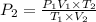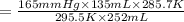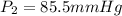Chemistry, 30.03.2020 16:52 haelleydorethy
Two gas-containers, A and B, are connected with a valve. The first container, A, has a volume of 135 mL, and the second container, B, has a volume of 117 mL. A sample of gas originally in container A is at 22.5 C and the pressure is 165 mmHg. What is the pressure (in mmHg) of the gas sample when the valve is opened and the gas now occupies both containers at a temperature of 12.7 C

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1


Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
Two gas-containers, A and B, are connected with a valve. The first container, A, has a volume of 135...
Questions


Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00


English, 12.02.2021 14:00


Advanced Placement (AP), 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00




Mathematics, 12.02.2021 14:00


Mathematics, 12.02.2021 14:00




English, 12.02.2021 14:00

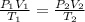
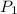 = initial pressure of gas in container A = 165 mmHg
= initial pressure of gas in container A = 165 mmHg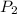 = final pressure of gas = ?
= final pressure of gas = ?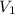 = initial volume of gas in container A=
= initial volume of gas in container A= 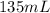
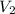 = final volume of gas = 135 mL + 117 mL = 252 mL
= final volume of gas = 135 mL + 117 mL = 252 mL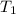 = initial temperature of gas in container A =
= initial temperature of gas in container A = 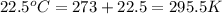
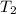 = final temperature of gas =
= final temperature of gas =