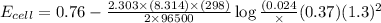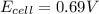In a test of a new reference electrode, a chemist constructs a voltaic cell consisting of a Zn/Zn2+ half-cell and an H2/H+ half-cell under the following conditions: [Zn2+ ] = 0.024 M [H+ ]= 1.3 M partial pressure of H2 = 0.37 atm. Calculate Ecell at 298 K (enter to 3 decimal places). Zn2+ (aq) + 2eLaTeX: -− LaTeX: \longrightarrow⟶ Zn(s) E° = LaTeX: -−0.76 V 2H+ (aq) + 2eLaTeX: -−LaTeX: \longrightarrow⟶ H2(g) E° = 0.00 V

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1


Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
In a test of a new reference electrode, a chemist constructs a voltaic cell consisting of a Zn/Zn2+...
Questions



Biology, 12.03.2021 05:30



Mathematics, 12.03.2021 05:30

English, 12.03.2021 05:30



English, 12.03.2021 05:30

English, 12.03.2021 05:30




Advanced Placement (AP), 12.03.2021 05:30




Mathematics, 12.03.2021 05:30

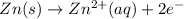
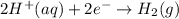
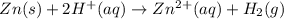
![E^o_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0570/4548/c67da.png)
![E^o_{[H^+/H_2]}=0.00V](/tpl/images/0570/4548/4b48a.png)
![E^o=E^o_{[cathode]}-E^o_{[anode]}](/tpl/images/0570/4548/51f3e.png)
![E^o=E^o_{[H^+/H_2]}-E^o_{[Zn^{2+}/Zn]}](/tpl/images/0570/4548/91ffc.png)
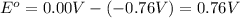
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]\times (p_{H_2})}{[H^+]^2}](/tpl/images/0570/4548/307e3.png)
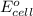 = electrode potential of the cell = ?
= electrode potential of the cell = ?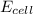 = emf of the cell = 0.76 V
= emf of the cell = 0.76 V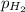 = 0.37 atm
= 0.37 atm![[Zn^{2+}]](/tpl/images/0570/4548/9c01a.png) = 0.024 M
= 0.024 M![[H^{+}]](/tpl/images/0570/4548/85507.png) = 1.3 M
= 1.3 M