
Chemistry, 30.03.2020 16:55 jc95704816
A liquid is exposed to infrared radiation with a wavelength of 6.92 × 10 − 4 cm. 6.92×10−4 cm. Assume that all the radiation is absorbed and converted to heat. How many photons are required for the liquid to absorb 58.28 J 58.28 J of heat?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
A liquid is exposed to infrared radiation with a wavelength of 6.92 × 10 − 4 cm. 6.92×10−4 cm. Assum...
Questions








Mathematics, 27.03.2020 22:50

Mathematics, 27.03.2020 22:50

Biology, 27.03.2020 22:50

Mathematics, 27.03.2020 22:50


Mathematics, 27.03.2020 22:50



English, 27.03.2020 22:50

Arts, 27.03.2020 22:50



Mathematics, 27.03.2020 22:50

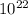 Photons
Photons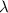 = 6.92 ×
= 6.92 × 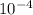 cm = 6.92 ×
cm = 6.92 × 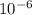 m
m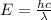
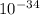 J - s
J - s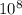
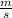
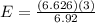 ×
× 
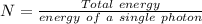
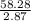 ×
× 

