
Chemistry, 30.03.2020 17:30 johnsonkia873
Write chemical equations and corresponding equilibrium expressions for each of the two ionization steps of carbonic acid. Part A Write chemical equations for first ionization step of carbonic acid. Express your answer as a chemical equation. Identify all of the phases in your answer. nothing Request Answer Part B Complete previous part(s) Part C Write chemical equations for second ionization step of carbonic acid. Express your answer as a chemical equation. Identify all of the phases in your answer. nothing

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Write chemical equations and corresponding equilibrium expressions for each of the two ionization st...
Questions




Mathematics, 29.07.2019 09:30




Arts, 29.07.2019 09:30



French, 29.07.2019 09:30

Social Studies, 29.07.2019 09:30

Social Studies, 29.07.2019 09:30





English, 29.07.2019 09:30

Social Studies, 29.07.2019 09:30

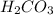 . It is a diprotic weak acid which means that it will release two hydrogen ions when dissolved in water
. It is a diprotic weak acid which means that it will release two hydrogen ions when dissolved in water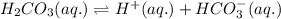
![Ka_1=\frac{[H^+][HCO_3^{-}]}{[H_2CO_3]}](/tpl/images/0570/5262/fb18f.png)
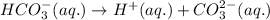
![Ka_2=\frac{[H^+][CO_3^{2-}]}{[HCO_3^-]}](/tpl/images/0570/5262/f465d.png)


