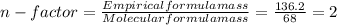A 5.769 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis and 14.92 grams of CO2 and 3.054 grams of H2O are produced. In a separate experiment, the molar mass is found to be 136.2 g/mol. Determine the empirical formula and the molecular formula of the organic compound.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1


Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

You know the right answer?
A 5.769 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis...
Questions

Mathematics, 08.11.2020 07:40

Mathematics, 08.11.2020 07:40

Mathematics, 08.11.2020 07:40

French, 08.11.2020 07:40

English, 08.11.2020 07:40

Computers and Technology, 08.11.2020 07:40

History, 08.11.2020 07:40



English, 08.11.2020 07:40

English, 08.11.2020 07:40


History, 08.11.2020 07:40

History, 08.11.2020 07:40


English, 08.11.2020 07:40

Chemistry, 08.11.2020 07:40

History, 08.11.2020 07:40

History, 08.11.2020 07:40

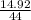 = 0.339 moles of carbonno. of moles of H₂O =
= 0.339 moles of carbonno. of moles of H₂O = 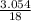 = 0.16 moles x 2 = 0.339 moles of hydrogenmass of carbon = 0.339 x 12 = 4.068 gmass of hydrogen = 0.339 x 1 = 0.339 gmass of oxygen = 5.769 - (4.068 + 0.339)
= 0.16 moles x 2 = 0.339 moles of hydrogenmass of carbon = 0.339 x 12 = 4.068 gmass of hydrogen = 0.339 x 1 = 0.339 gmass of oxygen = 5.769 - (4.068 + 0.339)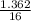 = 0.085 moles
= 0.085 moles 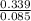 = 4relative mole ratio for hydrogen =
= 4relative mole ratio for hydrogen = 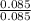 = 1 empirical formula C₄H₄Oempirical formula mass = 4 x 12 + 4 x 1 + 1 x 16 = 68molecular mass = 136.2 g / mol
= 1 empirical formula C₄H₄Oempirical formula mass = 4 x 12 + 4 x 1 + 1 x 16 = 68molecular mass = 136.2 g / mol