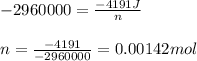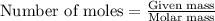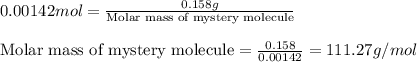Chemistry, 30.03.2020 18:52 Herbie3070
0.158 g of a mystery molecule is placed into a bomb calorimeter that has a heat capacity of 1650 J/C. After the sample is combusted the temperature of the calorimeter increased by 2.54 C. Determine the molar mass of the mystery molecule if the enthalpy of combustion for one mole is 2960 kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1


Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3
You know the right answer?
0.158 g of a mystery molecule is placed into a bomb calorimeter that has a heat capacity of 1650 J/C...
Questions


History, 28.01.2021 18:20

Mathematics, 28.01.2021 18:20

Mathematics, 28.01.2021 18:20




Computers and Technology, 28.01.2021 18:20


Mathematics, 28.01.2021 18:20

English, 28.01.2021 18:20






Mathematics, 28.01.2021 18:20



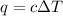
 = change in temperature =
= change in temperature = 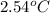
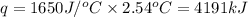
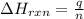
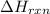 = enthalpy change of the reaction = -2960 kJ/mol = -2960000 J/mol (Conversion factor = 1 kJ = 1000 J)
= enthalpy change of the reaction = -2960 kJ/mol = -2960000 J/mol (Conversion factor = 1 kJ = 1000 J)