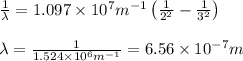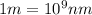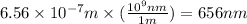Chemistry, 30.03.2020 18:45 rileyeddins1010
Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydrogen atom undergoes the transition from the energy level n = 3 n=3 to the level n = 2 .

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 11:10
Why would a doctor most likely restrict a patient's contact with other people while the patient receives internal radiation
Answers: 1
You know the right answer?
Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydroge...
Questions


Mathematics, 21.04.2020 05:54

Mathematics, 21.04.2020 05:54

Mathematics, 21.04.2020 05:54

History, 21.04.2020 05:54

Mathematics, 21.04.2020 05:54

History, 21.04.2020 05:54


English, 21.04.2020 05:54

Mathematics, 21.04.2020 05:54

Mathematics, 21.04.2020 05:54

English, 21.04.2020 05:54

Mathematics, 21.04.2020 05:54


Mathematics, 21.04.2020 05:54

Mathematics, 21.04.2020 05:54

Arts, 21.04.2020 05:54


Mathematics, 21.04.2020 05:54

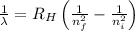
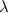 = Wavelength of radiation
= Wavelength of radiation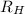 = Rydberg's Constant =
= Rydberg's Constant = 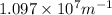
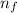 = Final energy level = 2
= Final energy level = 2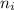 = Initial energy level = 3
= Initial energy level = 3