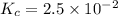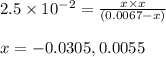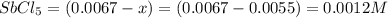Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Consider the reaction below. 0.0067 M SbCl5 is placed in a one liter flask and the system is allowed...
Questions


Mathematics, 20.05.2021 19:10

History, 20.05.2021 19:10




Mathematics, 20.05.2021 19:10

Mathematics, 20.05.2021 19:10


Arts, 20.05.2021 19:10

Spanish, 20.05.2021 19:10

Mathematics, 20.05.2021 19:10


Mathematics, 20.05.2021 19:10


English, 20.05.2021 19:10

Mathematics, 20.05.2021 19:10


Mathematics, 20.05.2021 19:10

Law, 20.05.2021 19:10

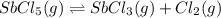
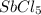 is 0.0012 M
is 0.0012 M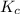 for above equation follows:
for above equation follows:![K_c=\frac{[Cl_2][SbCl_3]}{[SbCl_5]}](/tpl/images/0570/7430/32d06.png)