

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
For a certain diprotic acid, the pH at one half the volume to the first equivalence point is 4.15 an...
Questions

Mathematics, 11.05.2021 01:20


Mathematics, 11.05.2021 01:20



Mathematics, 11.05.2021 01:20

Mathematics, 11.05.2021 01:20

Mathematics, 11.05.2021 01:20


Mathematics, 11.05.2021 01:20

Social Studies, 11.05.2021 01:20




Mathematics, 11.05.2021 01:20


History, 11.05.2021 01:20



Social Studies, 11.05.2021 01:20

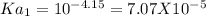 &
& 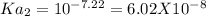
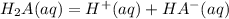 ----------------(i)
----------------(i)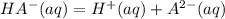 ---------------(ii)
---------------(ii)![Ka_{1}= \frac{[H^{+} ][HA^{-} ]}{[H_{2}A ]}](/tpl/images/0570/7557/d943f.png)
![pH_{1} = -logKa_{1} + log\frac{[Base]}{[Acid]}](/tpl/images/0570/7557/2ee84.png)
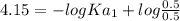
![pH_{2}=-log Ka_{2} + log\frac{[Base]}{[Acid]} \\pH_{2}=-log Ka_{2} + log\frac{[A^{2-} ]}{[HA^{-} ]}\\\\7.22 = - logKa_{2} +log(1)\\\\Ka_{2}=10^{-7.22}= 6.02 X10^{-8}](/tpl/images/0570/7557/9dab2.png)


