
Chemistry, 30.03.2020 19:07 turtlesage21
G For the reaction N2(g) + O2(g) --> 2 NO(g), the equilibrium constant, K=1.0x10-5 Which direction will the reaction move, and why, if the reactants and products have the following concentrations: [N2]=0.015 M [O2]=0.25 M [NO]=0.010

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
G For the reaction N2(g) + O2(g) --> 2 NO(g), the equilibrium constant, K=1.0x10-5 Which directio...
Questions

Biology, 23.11.2020 20:00



Mathematics, 23.11.2020 20:00

Mathematics, 23.11.2020 20:00

English, 23.11.2020 20:00

Mathematics, 23.11.2020 20:00


English, 23.11.2020 20:00

Mathematics, 23.11.2020 20:00


English, 23.11.2020 20:00

English, 23.11.2020 20:00



Mathematics, 23.11.2020 20:00

Social Studies, 23.11.2020 20:00

Mathematics, 23.11.2020 20:00


Chemistry, 23.11.2020 20:00

![\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0570/7732/10229.png) where the terms in the large bracket denote the concentrations of respective reacting species.
where the terms in the large bracket denote the concentrations of respective reacting species.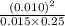 =
= 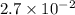
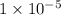 , we see that the Q is larger than that of K.
, we see that the Q is larger than that of K.

