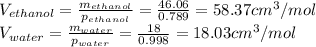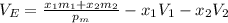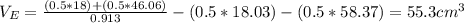Chemistry, 30.03.2020 19:16 hannah1571
The density of an equal-mass water 1 ethanol mixture is 0.913 g/cc at 20°C. If the density of water is 0.998 g/cm3 and ethanol is 0.789 g/cm3 with both at 20°C, does this equal-mass mixture possess a positive or negative excess volume at 20°C?

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1

Chemistry, 23.06.2019 14:00
During an acid-base titration, when do the contents of the beaker consist of only water, a salt, and a trace of indicator?
Answers: 2

Chemistry, 23.06.2019 15:30
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2
You know the right answer?
The density of an equal-mass water 1 ethanol mixture is 0.913 g/cc at 20°C. If the density of water...
Questions

Biology, 05.08.2019 00:30



Chemistry, 05.08.2019 00:30

Mathematics, 05.08.2019 00:30

History, 05.08.2019 00:30

Social Studies, 05.08.2019 00:30

Biology, 05.08.2019 00:30



English, 05.08.2019 00:30





Business, 05.08.2019 00:30

Mathematics, 05.08.2019 00:30


Mathematics, 05.08.2019 00:30