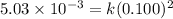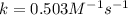Chemistry, 30.03.2020 19:12 SoccerHalo
Consider the reaction: 2A + B → C, and a kinetics study on this reaction yielded: [A] mol/L [B] mol/L Rate = mol/L/s 0.100 0.200 5.03 × 10−3 0.050 0.200 1.27 × 10−3 0.050 0.100 1.25 × 10−3 What is the value of the rate constant?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Consider the reaction: 2A + B → C, and a kinetics study on this reaction yielded: [A] mol/L [B] mol/...
Questions

Mathematics, 05.05.2020 04:56


Mathematics, 05.05.2020 04:56




Mathematics, 05.05.2020 04:56


Social Studies, 05.05.2020 04:56

Mathematics, 05.05.2020 04:56

Mathematics, 05.05.2020 04:56


Mathematics, 05.05.2020 04:57


Mathematics, 05.05.2020 04:57

Mathematics, 05.05.2020 04:57


Mathematics, 05.05.2020 04:57

Mathematics, 05.05.2020 04:57

English, 05.05.2020 04:57

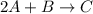
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0570/7972/10aeb.png)
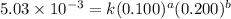 ....(1)
....(1)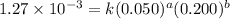 ....(2)
....(2)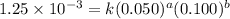 ....(3)
....(3)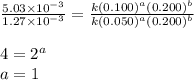
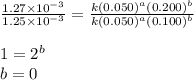
![\text{Rate}=k[A]^2[B]^0](/tpl/images/0570/7972/d2583.png)
![\text{Rate}=k[A]^2](/tpl/images/0570/7972/881f5.png)