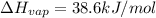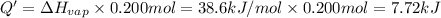Chemistry, 30.03.2020 21:38 angiejtc0908
G Ethanol boils at 78.4 °C with \DeltaΔHvap = 38.6 kJ/mol. A 0.200-mol sample of ethanol is heated from some colder temperature up to 78.4 °C, which requires 1.05 kJ of heat, and then vaporized. What will be the total amount of heat required (for both the heating and the vaporizing)?

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1
You know the right answer?
G Ethanol boils at 78.4 °C with \DeltaΔHvap = 38.6 kJ/mol. A 0.200-mol sample of ethanol is heated f...
Questions


Mathematics, 27.07.2021 02:40

Mathematics, 27.07.2021 02:40

Mathematics, 27.07.2021 02:40





Computers and Technology, 27.07.2021 02:40