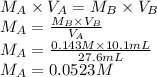Chemistry, 30.03.2020 19:38 cheaterman4121
A student is asked to standardize a solution of potassium hydroxide. He weighs out 1.08 g potassium hydrogen phthalate (KHC8H4O4, treat this as a monoprotic acid). It requires 36.8 mL of potassium hydroxide to reach the endpoint. A. What is the molarity of the potassium hydroxide solution? M This potassium hydroxide solution is then used to titrate an unknown solution of perchloric acid. B. If 10.1 mL of the potassium hydroxide solution is required to neutralize 27.6 mL of perchloric acid, what is the molarity of the perchloric acid solution? M

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
A student is asked to standardize a solution of potassium hydroxide. He weighs out 1.08 g potassium...
Questions

English, 03.11.2020 20:30

Chemistry, 03.11.2020 20:30


Mathematics, 03.11.2020 20:30

Social Studies, 03.11.2020 20:30


Geography, 03.11.2020 20:30

Biology, 03.11.2020 20:30

Mathematics, 03.11.2020 20:30




History, 03.11.2020 20:30

Health, 03.11.2020 20:30

Biology, 03.11.2020 20:30

Arts, 03.11.2020 20:30