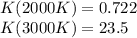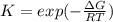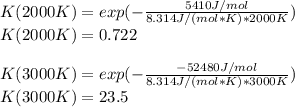The equation represents the decomposition of a generic diatomic element in its standard state. 1 2 X 2 ( g ) ⟶ X ( g ) Assume that the standard molar Gibbs energy of formation of X(g) is 5.41 kJ⋅mol − 1 at 2000. K and − 52.48 kJ⋅mol − 1 at 3000. K. Determine the value of K (the thermodynamic equilibrium constant) at each temperature. K at 2000. K = K at 3000. K =

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
The equation represents the decomposition of a generic diatomic element in its standard state. 1 2 X...
Questions





History, 05.09.2020 02:01




English, 05.09.2020 02:01



Mathematics, 05.09.2020 02:01


Mathematics, 05.09.2020 02:01




Advanced Placement (AP), 05.09.2020 02:01