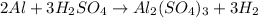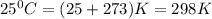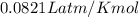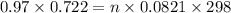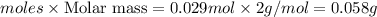G A sample that contains aluminum is reacted with sulfuric acid according to the equation given below. When the aluminum dissolved the total volume of gas collected over water at 25 °C is 0.722 L at a total pressure of 739 mm Hg. What mass of hydrogen gas is collected?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What are the concentrations of hydroxide and hydronium ions in a solution with a ph of 10.2?
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

You know the right answer?
G A sample that contains aluminum is reacted with sulfuric acid according to the equation given belo...
Questions


Mathematics, 11.07.2019 14:30



Business, 11.07.2019 14:30


Social Studies, 11.07.2019 14:30

Mathematics, 11.07.2019 14:30

Mathematics, 11.07.2019 14:30

Spanish, 11.07.2019 14:30

Spanish, 11.07.2019 14:30




English, 11.07.2019 14:30

Health, 11.07.2019 14:30


Spanish, 11.07.2019 14:30