
Chemistry, 30.03.2020 19:35 kaliemn7oyqxuy
A major component of gasoline is octane (C8H18). When liquid octane is burned in the air it reacts with oxygen gas to produce carbon dioxide gas and water vapor.
Calculate the moles of carbon dioxide produced by the reaction of 0.085 mol of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


You know the right answer?
A major component of gasoline is octane (C8H18). When liquid octane is burned in the air it reacts w...
Questions

Social Studies, 03.12.2021 16:20

Computers and Technology, 03.12.2021 16:20









History, 03.12.2021 16:20








Mathematics, 03.12.2021 16:20

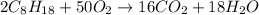
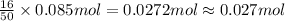 of carbon dioxide
of carbon dioxide 

